In trials the drug in combination with diet and exercise helped patients lose over 12 more weight than those who took a placebo along with. The FDA has now given the drug the all-clear.

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr
Immunization Action Coalition IAC.
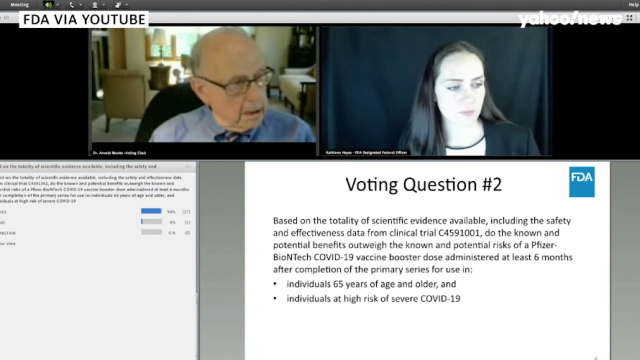
Fda booster recommendation. This recommendation supersedes previous Tdap recommendations regarding adults aged 65 years and older. An allergic reaction could occur after the vaccinated person leaves the clinic. There are two reasons that FDA could go against the recommendation Stifel analyst Paul Matteis wrote in a Dec.
Despite questions surrounding its efficacy the Food and Drug Administration on Monday approved a groundbreaking new medication that attacks the underlying Alzheimers disease process rather than treating just its symptoms writes The New York TimesIt is the first drug of its kind and the first new Alzheimers treatment in 18 years. The Pertussis Vaccines Work Group of ACIP reviewed the epidemiology of pertussis in adults aged 65 years and older and two cost-effectiveness models to assess the epidemiologic and economic impact of pertussis vaccination in this population. FDAs guidance documents including this guidance do not establish legally enforceable.
But no specific recommendation. Official recommendation position. The following is a transcript of an interview with former FDA Commissioner Scott Gottlieb that aired on Sunday July 4 2021 on Face the Nation.
Booster shots may not be necessary for another one to five years but companies are preparing to distribute them before the end of. Health experts say that any recommendation about booster shots should be made by public health officials at the CDC and FDA and not by pharmaceutical executives. Shares of messenger RNA-based vaccine makers fell on Wednesday as Centers for Disease Control.
Precision Vaccinations Johnson Johnson JJ announced it welcomes the recommendation by the World Health Organization WHO Strategic Advisory Group of Experts on Immunization for the Janssen Ebola vaccine regimen Zabdeno Ad26ZEBOV and Mvabea MVA-BN-Filo. 17 note to clients. The CDC Isnt Convinced Yet That Covid-19 Boosters Are Required.
If a Td booster vaccination is indicated during pregnancy ie more than 10 years since the previous Td vaccination then obstetriciangynecologists and other health care providers should administer the Tdap vaccine during pregnancy within the 2736-weeks-of-gestation window 7. Many of the testimonials and reviews on this site came from study participants or GOLO customers and members who followed the GOLO Metabolic Plan and also took the Release supplement. The Janssen Ebola vaccine therapy consists of two components.
What It Means for Vaccine Stocks. A recommendation from someone you know beats an algorithm any day. Timing of dosing primary and booster schedule and dosage range.
An increased possibility of administering booster doses in the near future. All recommendations about booster shots need to come from FDA. The FDA on Monday gave the drug accelerated approval based on evidence that it can reduce a likely contributor to Alzheimers rather than proof of a clear benefit against the disease.
This recommendation is because of the nonurgent nature of this. I support the ACIPs recommendation that the Johnson and Johnson COVID-19 vaccine be used for persons 18 years of age or older in the United States population under the FDA. A booster Covid-19 vaccine for people who have already been vaccinated may be needed as soon as eight to 12 months after their second shot according to.
While most Wall Street analysts doubt the FDA will approve the drug some are holding out the possibility of a surprise by the decision deadline of March 7. Arkansas jail dosing inmates with ivermectin in spite of stern FDA warnings. Zabdeno is given first and.
The GOLO weight loss system includes the GOLO Diet along with behavior and lifestyle recommendations including a recommendation for moderate exercise. For other signs that concern you call your health care provider. In addition the FDA approval ignored the recommendation of its outside advisors who said Biogen did not provide enough evidence of clinical.
Investors are breathing easier after the FDAs response to a potential link. There is still no federal public health recommendation for COVID-19 booster shots. If you see signs of a severe allergic reaction hives swelling of the face and throat difficulty breathing a fast heartbeat dizziness or weakness call 9-1-1 and get the person to the nearest hospital.
Oregon Health Authority Issues Statement On Fda Booster Dose Recommendation Ktvz

Fda Advisors Reject Pfizer Covid 19 Boosters For All But Agree To Shots For Elderly And High Risk Fiercepharma

Fda Recommends Covid 19 Booster Shots For Some Americans

Fda Panel Recommends Booster For Certain Groups Not General Public Supertalk Mississippi

Fda Panel Recommends Booster Shots For Over 65s Nhk World Japan News

Fda Panel Recommends Covid 19 Booster For Some Excludes Teens American Academy Of Pediatrics